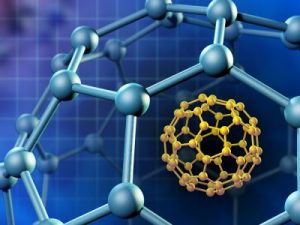
C60 VS GRAFEX® SUPER [C-60] FULLERENES.
Dr. Tom Bailey, Chief Scientist and Organic Chemist:
Comparison and characteristics between Grafex® Super[C-60] fullerene molecule and the Bucky Ball C60 fullerene molecule.
The following information is presented to introduce you to the newest fullerene-like molecule called Grafex® Super[C-60] , as well as giving you a well-researched history (see history page) of the comparison and characteristics between Grafex® Super[C-60] molecule and the Bucky Ball C60 molecule.
Both Grafex® Super[C-60] molecules and the Regular C60 molecules are made up cyclic (ring) structures which are commonplace throughout the natural world.
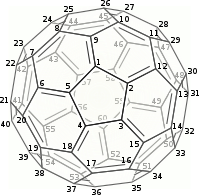
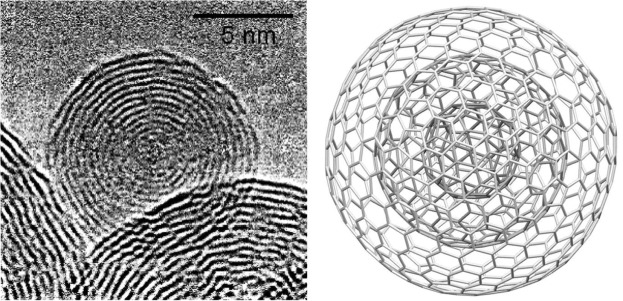
Regular C60 is a single layer of “60 carbon atoms” arranged in 20 hexagonal ring structures which are spherically arranged, creating a uniquely delocalized and diffuse electron pi (π) cloud that surrounds the entire Regular C60 molecule. This delocalized electron arrangement results in Regular C60’s stability and properties.
The Grafex® Super [C-60] molecule, on the other hand, is arranged in concentric or nested fullerene shells from 10 to 20 in count. The individual shells are then fused into a linear or branched polymer chain much like one would see in a string of pearls. Accordingly, Grafex® Super [C-60] “molecules” come in distinct individual molecules populated by “as many as 10 million carbon atoms on each molecule”. This presents a vastly larger number of carbon atoms available vs the mere 60 carbon atoms of the simple Regular C60 molecule. To better understand the level of quantium physis that we are working with, it has been reported that “one human cell” is make up of 100 trillion atoms.
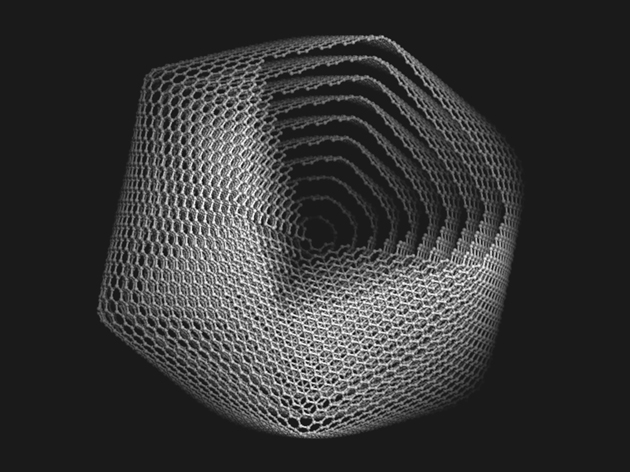
The Grafex® Super[C-60] molecule’s unique structure results in a significantly higher capacity of suppressing free radical activity with each shell exposed. It has a much greater number of sites on the outer shell to interact with damaging free radicals than the single shell Regular C60.
The Grafex® Super[C-60] molecule has also demonstrated anti–oxidant like properties that are far “superior” to that of the already amazing Regular C60 according to researchers that have used both. This is no small feat because Regular C60 has shown remarkable biocompatibility. This has resulted in a broad spectrum of possible medical applications and potential health benefits, despite earlier concerns of possible fullerene toxicity.
A popular explanation about these molecules has been that Regular C60 and the complex molecules-like Grafex® Super[C-60], are both “super antioxidants.” This was determined due to their amazing ability to ‘scavenge up free radicals” that attack cellular structures like enzymes, DNA, and membrane lipids. By binding with free radicals, fullerene molecules reduce the levels of free radical damage and their negative effects.
Amazingly this “scavenging” ability is “not a result” of a classical antioxidant action of giving a free radical the electrons it normally takes from other molecules. Fullerenes molecules unique structure and their affinity to take on electrons result in a different free radical interaction. In biological systems, they can also function as paradoxical electron acceptor-donor antioxidant molecules, to assist in neutralizing free radicals, as well as act as chaperones for detoxification enzymes.
The much larger and complex Grafex® Super[C-60] molecule has an exponentially larger capacity to assist the body whenever the body is under attack by very potent, excessive, or prolonged oxidative stress which can overwhelm the body’s natural defenses.
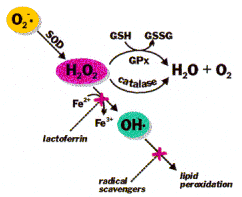
The body already possesses a potent free radical scavenging enzyme system to sense and regulate oxidative stress as well as stimulate gene transcription to replace and increase these important enzymes when necessary. By stimulating this system and binding with these proteins, fullerene molecules help by preserving and enhancing this inborn system.
This pathway is perhaps the most desirable way for the body to manage free radicals and oxidative stress. The body itself needs a limited amount of free radical activity and controlled oxidative stress which is central to disease resistance, cell survival, and overall health.
Grafex® Super[C-60]’s propensity to accept yet readily release electrons and protons may effectively allow it to function as an electron or proton sink to aid in certain charge transfer or biochemical reactions in the cell.
The advantages of Grafex® Super[C-60]’s greater capabilities over the diminutive Regular C60 carries over into other possible ways that fullerene molecules can lead to beneficial effects in living systems. Among these are positive impacts on the powerhouses of the cell, the mitochondria.

Fullerenes molecules are both protective and restorative to the impaired mitochondria that are struggling to produce energy in the cell during and after injury or excessive and prolonged stress. The impact on the restoration and return to normal energy production, free radical regulation and signaling, as well as DNA stabilization, transcription, and intercellular communication, is highly beneficial for the return to normal health.,
This may help explain reports by some cancer patient users of Grafex® Super[C-60], as to why they have little or no side effects from chemotherapy agents that are normally highly toxic to the liver and kidneys.
Considering as one researcher put it “(preliminary result): “Grafex® Super[C-60’s] nano-onions are at least an order of magnitude (10 times) better at neutralizing free radicals than C60 Fullerene molecules”.
A thorough mechanism on how fullerene molecules are used and metabolized in the body is still not well understood and further scientific research effort is needed for clarification. Nevertheless, we do know that fullerene molecules bind to proteins enhancing their hydration with polarized structured water layers. This structured cell water stabilizes and influences their folding; it also facilitates dynamic electron charge transfer activity, thereby helping the cell to function better. It is perceived that this molecule may well be a preventor of diseases and/or future health problems, however, that would be a very difficult factor or situation to verify or prove.
As an interesting side note, the discovery of the MRI was based on the fact that diseased cells contained less structured water than healthy cells.
While the free radical scavenging action of fullerene molecules can be tremendously important to cell health in our very toxic world; this may not be the full story.
Besides interacting with the free radical themselves, fullerene molecules can also help the body to preserve and increase its natural resistance to free radicals and oxidative stress.
Additionally, the beneficial protein interaction very likely extends to all active proteins in the body. This may be seen in the reduction of inflammation, activation of stem cells, and even help explain the painless dissolution and safe elimination of kidney stones (personal reviews) reported by some users of Grafex® Super[C-60].
Additional positive testing was achieved with the Grafex® Super[C-60]molecule in a published research study conducted by Dr. Desantis at the University of New York, on genetically engineered mice, as well as a non-published internal study by GNO, LLC using normal pet-store mice.
Exceptional health and vigor, absence of tumor development and survival well past the normal life expectancy of these mice, were again observed in these studies. (see mice studies) Yet, an explanation for these effects eludes our efforts and the science behind them is still a mystery.
As far back as 2009, a study by Zeynalov, et. al. in evaluating the radical scavenging efficiency of different Fullerenes C60, C70 and fullerene soot, showed that Regular C60 fell far behind both in antioxidant activity.
Another inconvenient fact is that Fullerenes have a strong affinity for electrons and behave as electron deficient alkenes interacting with other molecules by addition reactions. They are definitely not reducing agents in any classical sense.

Does this mean that Regular C60 is NOT an awesome molecule and is NOT a free radical scavenger? No, and No! Regular C60 revolutionized the scientific world when it was discovered and as we learned more about C60 and its molecular structure; the more scientist became amazed at its wonderful properties.
The take away here is that the same molecular structure that conveys Regular C60 its wondrous potential is present in other Fullerenes. Furthermore, it is potentiated considerably in larger, more complex, multi-shell fullerene material to the point of inducing additional beneficial properties and characteristics.
It is of interest to note that many websites that offer Regular C60 and C60 products for sale take pains to emphasize their C60 purity. It has recently been pointed out that currently there is no known production process that delivers pure C60 without the use of some type of an organic solvent. The purpose of the solvent is to remove non-C60 carbon which is a by-product of all known production processes.
Well… need I say more? Without getting technical, that’s the scope of the comparison between Regular C60 and Grafex® Super[C-60].
An article titled Solubility of Light Fullerenes in Vegetable Oils was published by Semenov in 2009 and is well worth reading. Below is a short paragraph as to his reasearch findings:
“Standard fullerenes obtained as a rule from solutions in aromatic solvents (toluene, xylenes, dichlorobenzenes, etc.) inevitably contain remains of these solvents. Even after an hours-long high-temperature (200–250°С) drying in sufficiently high vacuum (0.01 mm Hg) the remaining content of solvents is from thousandth up to hundredth parts of wt %.
The alternative complete removal of admixed solvents can be reached probably only by a vacuum high-temperature sublimation of fullerene at very low residual pressures (<10 mm Hg).
Experiments have shown that such a process is rather expensive and labor-consuming. For example, the price for so-called “sublimated fullerene С60” free from admixed solvents, is 2–4 times higher than the price for “ordinary” fullerene С60 obtained by the standard procedure of drying in a vacuum box.”
Any site claiming a proprietary solvent-free Regular C60 product should provide scientific confirmation that their production process and Regular C60 products are solvent free. Furthermore, it was noted that color is no guarantee of quality, purity, and or effectiveness of the C60 product.
The production of Grafex® Super[C-60] does not require the use of solvents of any kind, nor is it used to achieve its very pure, high quality fullerene material. The evidence of this has been presented in detail on this website. And the research into the characteristics, properties, and most importantly, its toxicity and biocompatibility are extensive and unmatched by any other pristine fullerene product that has been publicly published. (see toxicity testing)
When one begins to consider these things, it is harder to understand why someone would choose a product containing a single shell Regular C60 molecule material over a complex, multi-shell fullerene material such as Grafex® Super[C-60].
Free radicals come in many shapes, sizes, and chemical configurations. What they all share is a huge appetite for electrons, stealing them from any nearby substances that have them available. This electron theft can radically alter the “loser’s” structure or function. It has been reported that free radical damage can change the instructions coded in a strand of DNA. It can make a circulating low-density lipoprotein (LDL, sometimes called bad cholesterol) molecule more likely to get caught or stuck in an artery wall. Or it can also alter a cell’s membrane, changing the flow of what enters the cell and what leaves it. Humans are not defenseless against free radicals. Your body, being used to this relentless attack, can and does makes scads of molecules that quench free radicals as surely as water can douse a fire. We also extract free-radical fighters from food. These molecules are often lumped together as “antioxidants.” They work by generously giving electrons to free radicals without turning into electron-scavenging substances themselves.
There are hundreds, probably thousands, of different molecular substances that can act as antioxidants. The most familiar ones are vitamin C, vitamin E, beta-carotene, and other related carotenoids, along with the minerals selenium and manganese. They’re joined by glutathione, coenzyme Q10, lipoic acid, flavonoids, phenols, polyphenols, phytoestrogens, and many more.
But using the term “antioxidant” to refer to molecular substances is misleading. It is really a chemical property, namely, the ability to act as an electron donor. Some substances that act as antioxidants in one situation may be prooxidants—electron scavengers—in a different chemical arena. Another big misconception is that antioxidant molecules are interchangeable. They are not. Each and every one has its own unique chemical behaviors and biological properties. They almost certainly evolved as parts of elaborate networks, with each different molecular substance playing slightly different roles.
