GC – Gas chromatography is used in analytical chemistry for separating an analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture.
The combination of Gas Chromatography(GC) and Mass Spectrometry(MS) is an invaluable tool in the identification of Polycyclic Aromatic Hydrocarbons (PAHs) which are a group of over 100 different chemical molecules.
Gas Chromatography(GC)
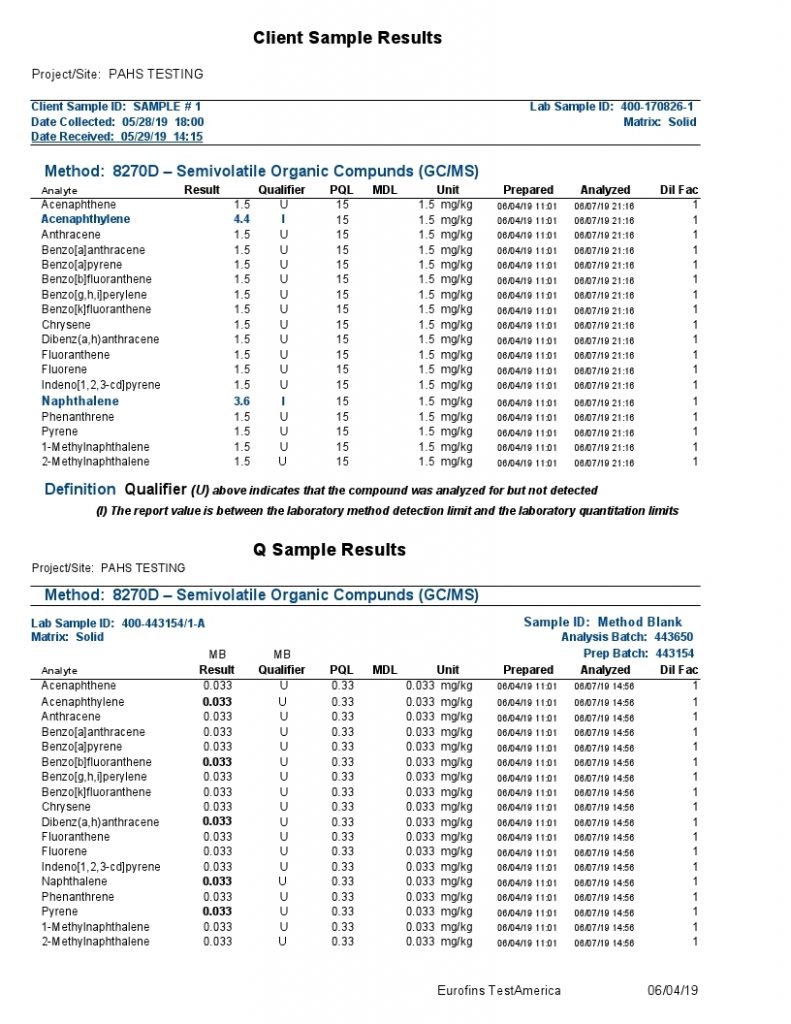
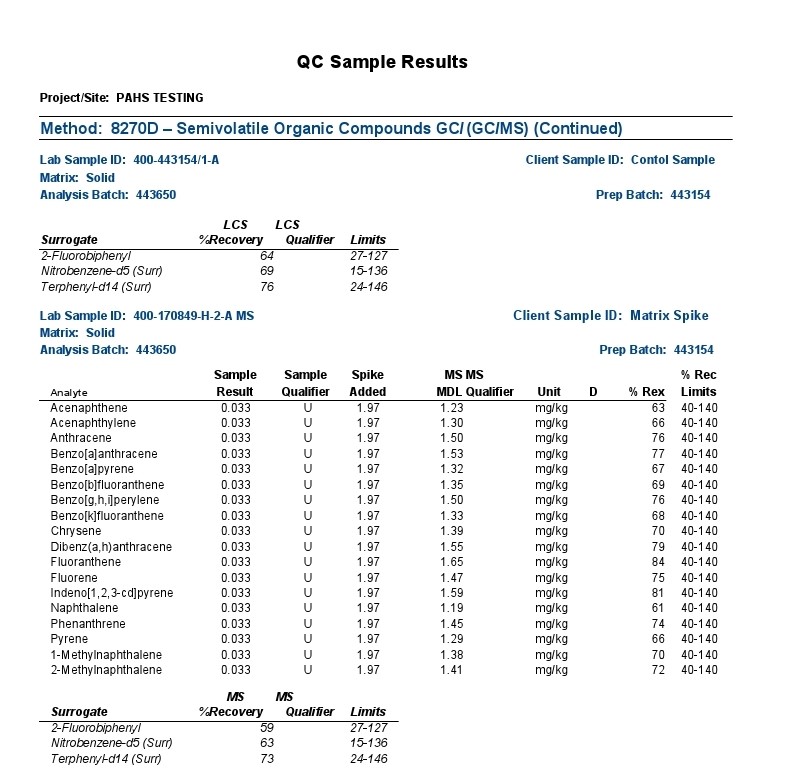
The polycyclic aromatic hydrocarbons (PAHs) listed above were the focus of a most rigorous and reproducible analysis by a certified laboratory strictly following standardized testing protocols.
Definition Qualifier:
(U) above indicates that the compound was analyzed for but not detected
(I) The report value is between the laboratory method detection limit and the laboratory quantitation limit.
Since its discovery at Rice University in 1985, C60 fullerene has been found to accompany the formation of soot that likewise often bears polycyclic aromatic hydrocarbons (PAHs) of a generally perceived unhealthful nature.
Accordingly, a lengthy list of PAHs provided in the above gc-ms analytical report were evaluated for presence in the Grafex® Super [C-60] by a highly reputable certified laboratory utilizing a most rigorous and reproducible procedure following well-established standardized testing protocols.
Analysis by gc-ms involves the separation and resolution of the various PAH components utilizing a specialized capillary tube a third of a football field in length (30 meters). As each resolved component exits the gas chromatographic (gc) column or capillary tube, it is ionized via a mass spectrometric (ms) hookup and detected electronically.
As with most any sophisticated instrumentation, electronic detection of a signal is required against a baseline signal often characterized as electronic noise. In the above report, the background noise is insufficiently resolved from a true signal with most signals presented as undetectable (“U”) at the 1.5 ppm MDL (Method Detection Limit); the 4.4 & 3.6 ppm results for acenaphthalene and naphthalene respectively are presented with an “I” designation suggesting also poor distinction from background electronic noise and the laboratory practical quantification limit (limitations associated with the laboratory analytical sample preparation).
So, all results are tied to an electronic signal inseparable and indistinguishable beyond the electrical noise of the instrumentation and/or the reproducibility of analytical sample preparations according to the labels “U” and “I” explained by the testing company.
Accordingly, no measurable PAH content in the nanocarbon material was detected in the low ppm limits of the analytical procedure. These results correspond with the CHN analysis also provided at this website from Micro-Analysis, Inc. where no hydrogen content was detected thereby likewise confirming that no PAH content is detected.
Dr. Tom Bailey
Chief Scientist and Organic Chemist
